Our latest publications are listed below, and can be filtered by research topic as listed under the Research Section.
A thermodynamic closure for the simulation of multiphase reactive flows (2019)
A simple thermodynamic closure for the simulation of multiphase reactive flows is presented. It combines a fully explicit thermodynamic closure appropriate for weakly thermal multiphase flow simulations, with the classical variable heat capacity ideal gas thermodynamic closure, commonly used for reactive flows simulations. Each liquid and gas component is assumed to follow the recent Noble-Abel Stiffened Gas equation of state, fully described by a set of five parameters. A new method for setting these parameters is presented and validated through comparisons with NIST references. Comparisons with a well-known cubic equation of state, Soave-Redlich-Kwong, are also included. The Noble-Abel Stiffened-Gas equation of state is then extended as to cope with variable heat capacity, to make the mixture thermodynamic closure appropriate for multiphase reactive flows.
Details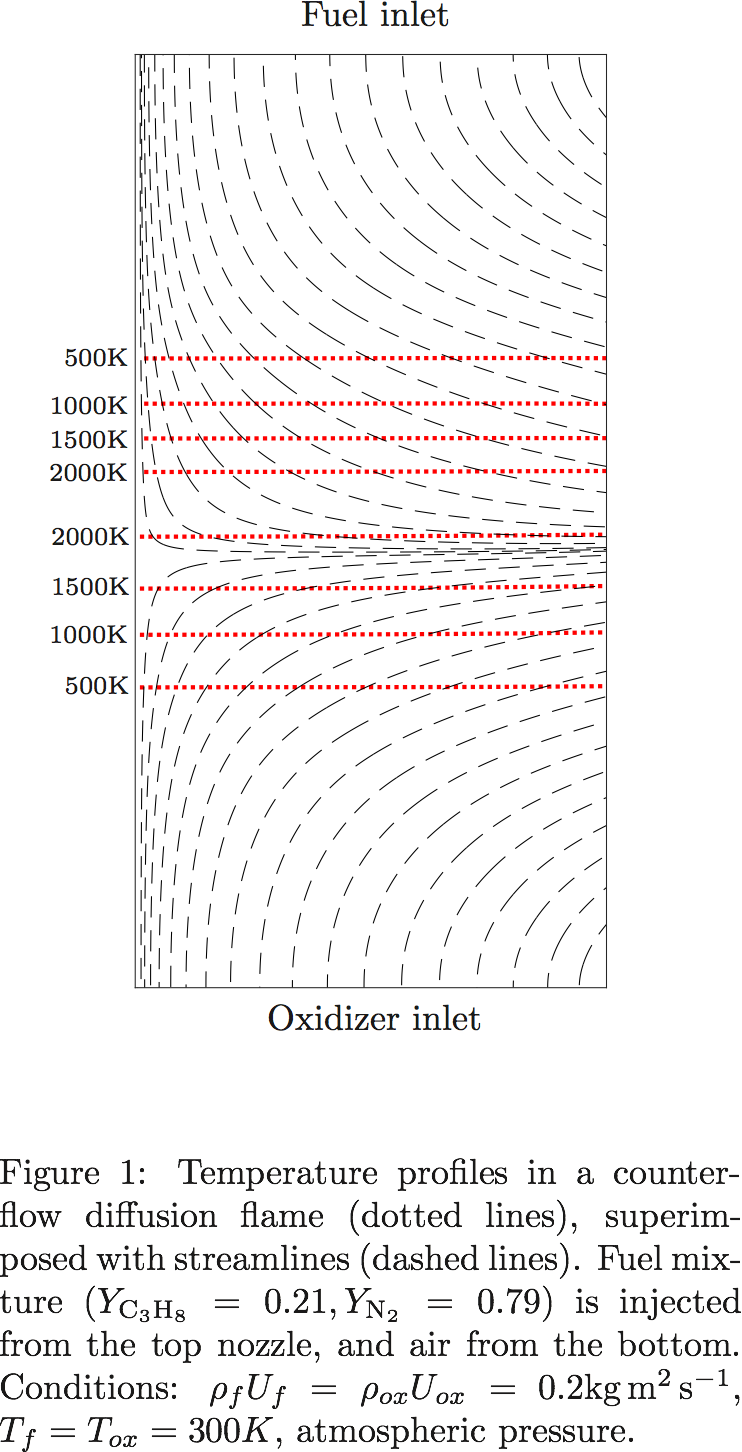
A Lattice-Boltzmann model for low-Mach reactive flows (2018)
A new Lattice-Boltzmann model for low-Mach reactive flows is presented. Based on standard lattices, the model is easy to implement, and is the first, to the authors’ knowledge, to pass the classical freely propagating flame test case as well as the counterflow diffusion flame, with strains up to extinction. For this presentation, simplified transport properties are considered, each species being assigned a separate Lewis number. In addition, the gas mixture is assumed to be calorically perfect. Comparisons with reference solutions show excellent agreement for mass fraction profiles, flame speed in premixed mixtures, as well as maximum temperature dependence with strain rate in counterflow diffusion flames.
DetailsExtension of a wide-range three-step hydrogen mechanism to syngas (2018)
Previously we have shown [1] how a single species X can be introduced, representing either HO2 for high-temperature ignition or H2O2 for low-temperature ignition, to develop an algorithm that covers the entire range of ignition, flame-propagation, and combustion conditions, without a significant degradation of accuracy, for hydrogen-air systems. By adding relevant CO chemistry to the hydrogen chemistry, this same approach can be applied to derive a comparably useful four-step reduced-chemistry description for syngas blends that have small enough concentrations of methane, other hydrocarbons, or other reactive species to be dominated by the elementary steps of the H2/CO system. The present communication reports the resulting extended algorithm. [1] P. Boivin, C. Jiménez, A. L. Sánchez, and F. A. Williams, “A four-step reduced mechanism for syngas combustion,” Combustion and Flame, vol. 158, no. 6, pp. 1059–1063, 2011.
Details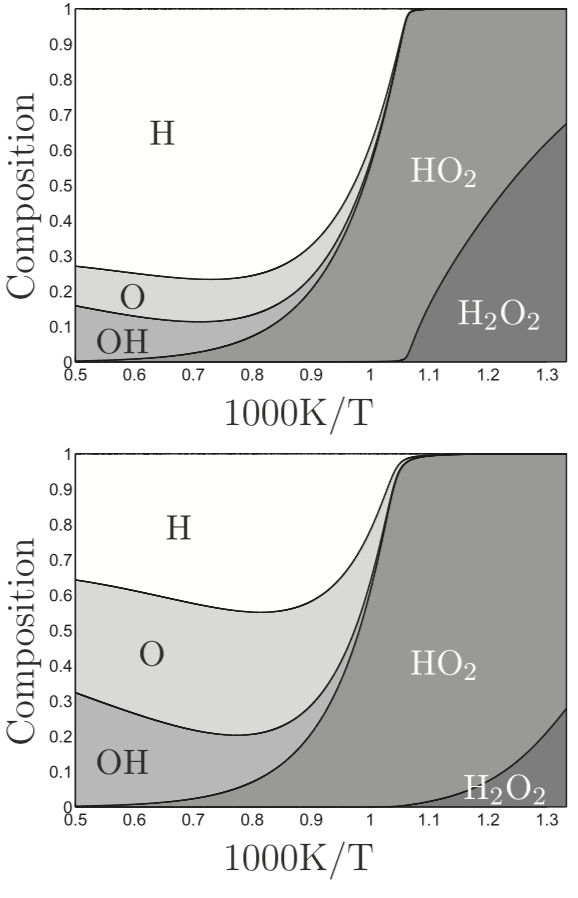
Analytical prediction of syngas induction times (2017)
Computations indicate that, under all possible conditions of practical interest, including temperatures both above and below the crossover temperature at which the rates of the H2 + O2 branching and termination steps are equal, twelve irreversible elementary steps suffice to provide accurate values of induction times in autoignition processes of fuels consisting of mixtures of H2, CO, and inerts. At high temperatures, this time is controlled by the time required for the radical pool to reach a steady state, with heat release being negligible during that time. This time is approximated well by the time that it would take for HO2 to reach a steady state if its consumption rate were dominated by formation of H2O2 at all temperatures, as it is at low temperatures. Below the crossover temperature, the time to reach an HO2 steady state becomes shorter than the induction time, and the heat release becomes non-negligible once HO2 has reached a steady state, resulting in the induction time progressively approaching that of a thermal explosion, which includes an effectively autocatalytic production of H2O2. On the basis of these observations, analytical approximations are introduced here that enable induction times to be calculated accurately under all conditions.
Details